A Pre-Antibiotic World Is Not an Option: Lessons from the Past
Written on
The Threat of Antibiotic Resistance
Antibiotic-resistant bacterial strains are increasingly appearing, and the World Health Organization warns that by 2050, these "superbugs" could lead to up to 10 million deaths each year. What measures can we take to avert this looming crisis?

When the Covid-19 pandemic unfolded, a surge of biological discussions emerged across media platforms. Health professionals and scientists were actively explaining the mechanisms behind epidemics and pandemics, emphasizing the importance of proactive strategies to mitigate their devastating effects.
Potential future scenarios, particularly during the somber lockdown periods, included the revival of ancient pathogens due to thawing permafrost and the alarming possibility of reverting to a pre-antibiotic landscape fueled by the rise of resistant bacteria. This latter prospect may be nearer than we think.
The Accidental Discovery of Antibiotics
"One sometimes finds what one is not looking for. When I woke up just after dawn on that September day, 1928, I certainly didn’t plan to revolutionize all medicine by discovering the world’s first antibiotic, or bacteria killer. But I suppose that was exactly what I did."
— Alexander Fleming on the discovery of penicillin
In September 1928, microbiologist Alexander Fleming returned to his laboratory in London to a troubling sight: mold contaminating his bacterial cultures. While most scientists would have discarded the contaminated samples, Fleming noted that the bacteria did not thrive near the mold, suggesting that it produced a substance harmful to them. This observation led to the discovery of penicillin, marking the dawn of the antibiotic age.
Despite recognizing the significance of his findings, Fleming's discovery initially went unnoticed until the late 1930s when researchers at Oxford developed a method to efficiently purify penicillin.
Antibiotics and Their Limitations
Unfortunately, antibiotics are not a permanent solution. As bacteria evolve alongside us, they develop resistance to our treatments. Unlike viruses, which are simpler in structure, bacteria are complex organisms. This complexity allows us to target their unique features with antibiotics.
The side effects of antibiotics stem not from damage to our cells but from the disruption of our beneficial gut microbiota. Bacterial reproduction involves DNA replication followed by cell division, enabling rapid population growth. During this process, mutations can occur, some of which may confer resistance to antibiotics.
When an antibiotic is used, it exerts selective pressure, eliminating non-resistant bacteria and allowing resistant strains to thrive.
The Realities of a Pre-Antibiotic Era
Antibiotic resistance is a pressing issue today, with an alarming rise in multi-drug resistant strains, including Staphylococcus aureus. The emergence of resistant strains has increased dramatically over recent decades. For example, ceftaroline was introduced in 2010, but resistant strains were identified the very next year.
The WHO projects that, at the current rate, antibiotic resistance could account for approximately 10 million deaths annually by 2050—far surpassing the 3 million deaths attributed to Covid-19 in 2020. While bacteria spread more slowly than viruses, many healthcare systems are ill-equipped to handle an outbreak of resistant strains. Returning to a pre-antibiotic world would complicate even routine medical procedures.
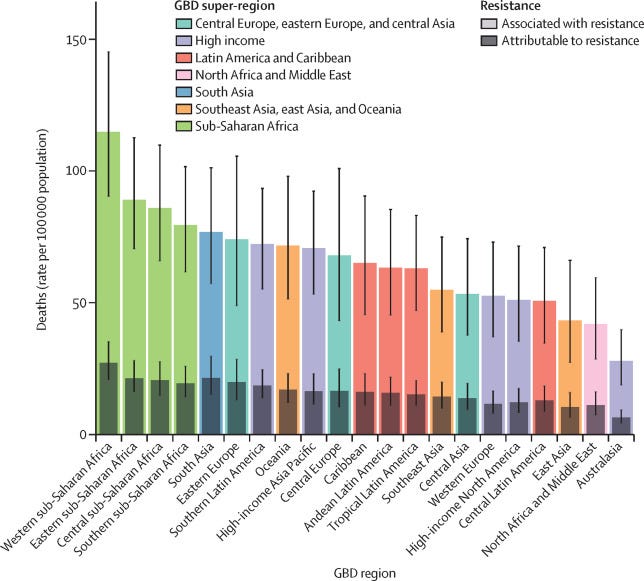
The link between drug exposure and resistance is clear, and the overuse of antibiotics—often due to incorrect prescriptions and extensive agricultural practices—only accelerates this problem.
On the Development of New Antibiotics
It may come as a shock that the rate of new antibiotic introductions has declined since the 1990s. This is largely due to the reliance on private companies for drug development, as antibiotics are not particularly lucrative. They are typically used for short durations and quickly resolve infections, unlike medications for chronic conditions.
In 2013, a cost-benefit analysis indicated that a new antibiotic is valued at around $50 million, whereas drugs for chronic illnesses can reach about $1 billion. Additionally, new antibiotic resistance will inevitably emerge. However, responsible antibiotic use can delay this process, allowing time to develop new treatments.
Exploring Collateral Sensitivity
Recent research published in Nature Communications explored a phenomenon called collateral sensitivity (CS), which could provide an alternative strategy for combating bacteria without relying on new antibiotics.
CS occurs when bacteria resistant to one antibiotic are more vulnerable to another. The challenge is to avoid selecting for new resistant strains. By utilizing current knowledge of the molecular basis of antibiotic resistance, scientists propose that temporary induction of resistance can enhance susceptibility to other antibiotics.
In their study, researchers demonstrated that strains of the multi-resistant bacterium Pseudomonas aeruginosa could be treated effectively by exploiting CS. They found that transiently inducing resistance allowed for treatment without promoting further resistance.
While still in the experimental stage, these results offer a promising avenue in the battle against bacterial resistance.
The Responsibility to Act
Advancements in science and technology have granted us a comfort we often take for granted. Just as we must vigilantly oversee socio-political systems, we should also monitor our interactions with the natural world, including our relationship with microorganisms and climate stability.
The Covid-19 pandemic served as a stark reminder of how fragile our systems can be. We must adopt a long-term perspective and proactive measures to prevent foreseeable crises.
Antimicrobial resistance, like climate change, has been a topic of concern for years. Yet, the slowdown in the production of new antibiotics indicates a broken market that relies heavily on private investment. This reality calls for a public long-term strategy to address healthcare emergencies.
Our instincts may not immediately recognize the threats posed by complex phenomena such as disease outbreaks and climate change, often reacting only when it’s too late. The lessons from Covid-19 and climate change compel us to take action now.
The first video titled Antibiotics: Cherish or Perish features Dr. Elizabeth Hermsen discussing the delicate balance we must maintain with antibiotic use and the potential consequences of misuse.
The second video, Antibiotics: The Surprising Truth About Probiotics and What to Do Instead, explores the relationship between antibiotics and probiotics, offering insights into managing antibiotic use effectively.