Understanding Why Clouds Stay Afloat in the Sky
Written on
Chapter 1: The Nature of Clouds
Clouds consist of a multitude of minuscule water droplets that drift within the atmosphere. Each droplet typically measures between 3 to 10 micrometers in diameter (1 micrometer = 1×10⁻⁶ m). Despite their small size, these droplets possess a density comparable to that of liquid water, which is significantly greater than that of the surrounding air. This leads to a curious question: why don’t clouds simply descend from the sky?
The explanation is more straightforward than one might think:
- Clouds contain tiny water droplets as well as water vapor.
- Water droplets are considerably denser than air.
- Water vapor is less dense than air.
- When water droplets and vapor are combined in the right ratios, the overall density of the cloud matches that of the surrounding air, allowing it to float without descending.
Section 1.1: The Density of Water Droplets
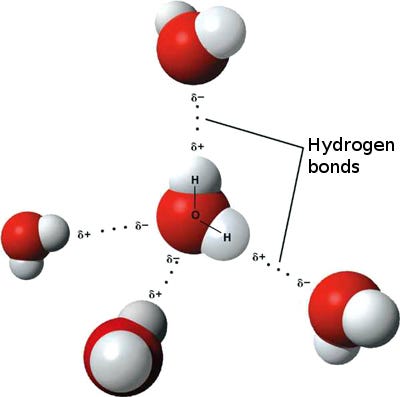
Water droplets are indeed much denser than air. Unlike a glass of water that remains grounded, liquid water is substantially heavier compared to air, which is gaseous in nature. This difference arises from the hydrogen bonds that hold water molecules together.
Section 1.2: The Lightness of Water Vapor
In contrast to liquid water, water vapor exists as individual molecules. Each water molecule has a molecular weight of 18 g/mol, which is lighter than the average molecular weight of air at approximately 29 g/mol. This is significant, as the density of water vapor is lower than that of air.
Air consists of a mixture of gases, primarily:
- 78.1% nitrogen (N₂)
- 20.9% oxygen (O₂)
- 0.934% argon (Ar)
- 0.033% carbon dioxide (CO₂)
Along with trace amounts of other gases such as neon (Ne) and methane (CH₄). To calculate the average molecular weight of air, one can use the following formula:
28 g/mol (N₂) × 0.781 +
32 g/mol (O₂) × 0.209 +
40 g/mol (Ar) × 0.00934 + … ≈ 29 g/mol (Air)
Section 1.3: The Balance of Density
When water droplets and vapor are mixed in a specific ratio, the density of the cloud can equal that of the air surrounding it. The relationship can be summarized as follows:
Water vapor (gaseous water) < Air << Water droplets (liquid water)
This means that if a lighter substance (water vapor) is combined with a heavier substance (water droplets), the resulting mixture can achieve a density comparable to that of air. Consequently, clouds remain suspended because they share the same density as the air around them.
Chapter 2: Conclusion
Understanding these principles sheds light on the remarkable phenomenon of clouds floating gracefully in our atmosphere.
SOCIAL: